
Comparison of the performance of exact-exchange-based density functional methods: The Journal of Chemical Physics: Vol 137, No 11
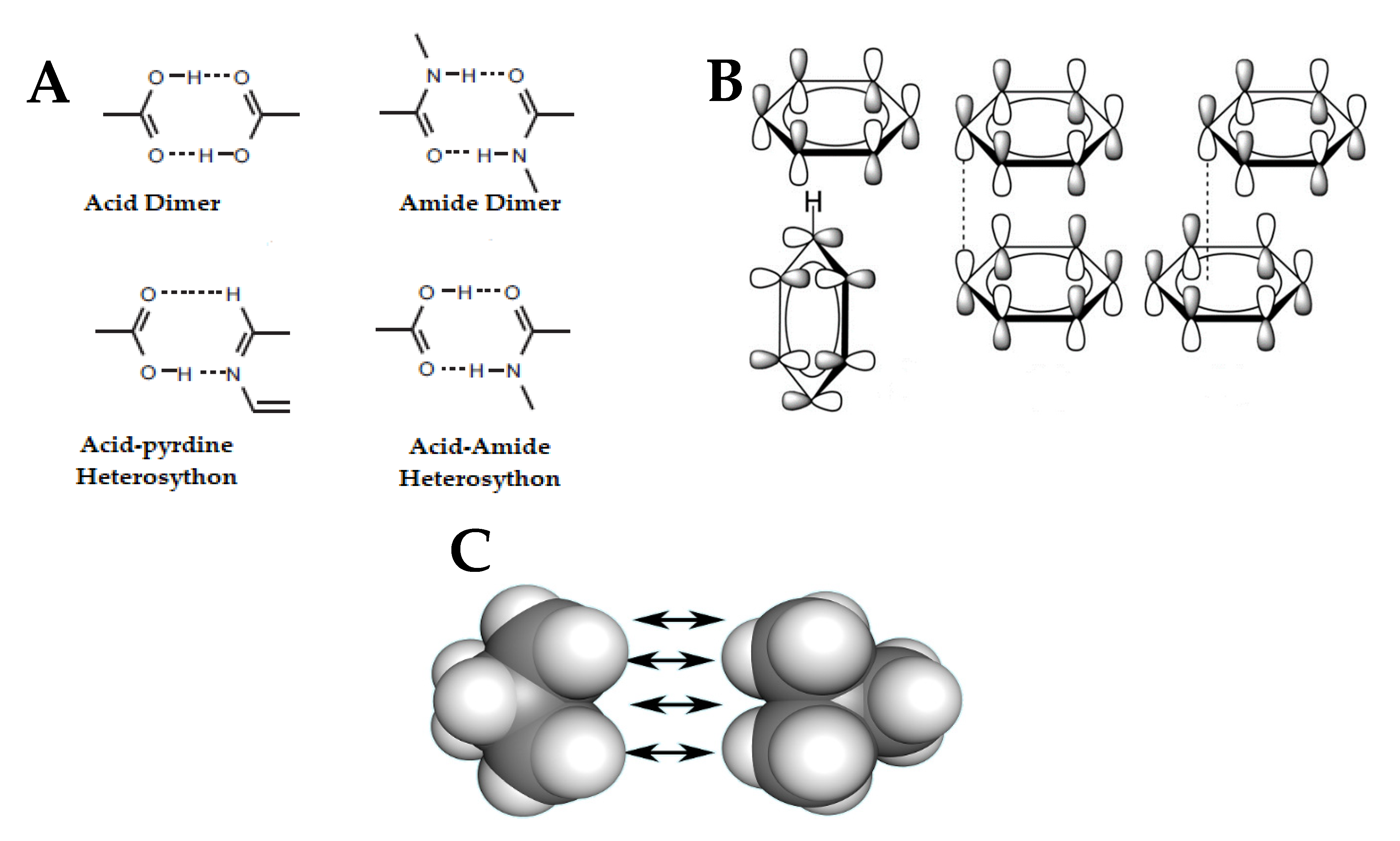
Crystals | Free Full-Text | Nano- and Crystal Engineering Approaches in the Development of Therapeutic Agents for Neoplastic Diseases | HTML
PDF) Hydrogen bonding between histidine and lignin model compounds or redox mediators as calculated with the DFT method. Effects on the ease of oxidationElectronic supplementary information (ESI) available: Fig. S1: comparison of

The C4H6•+ Potential Energy Surface. 1. The Ring-Opening Reaction of Cyclobutene Radical Cation and Related Rearrangements | Journal of the American Chemical Society

H2 Dissociation on H-Precovered Ni(100) Surface: Physisorbed State and Coverage Dependence,The Journal of Physical Chemistry C - X-MOL

Describing static correlation in bond dissociation by Kohn–Sham density functional theory: The Journal of Chemical Physics: Vol 122, No 9
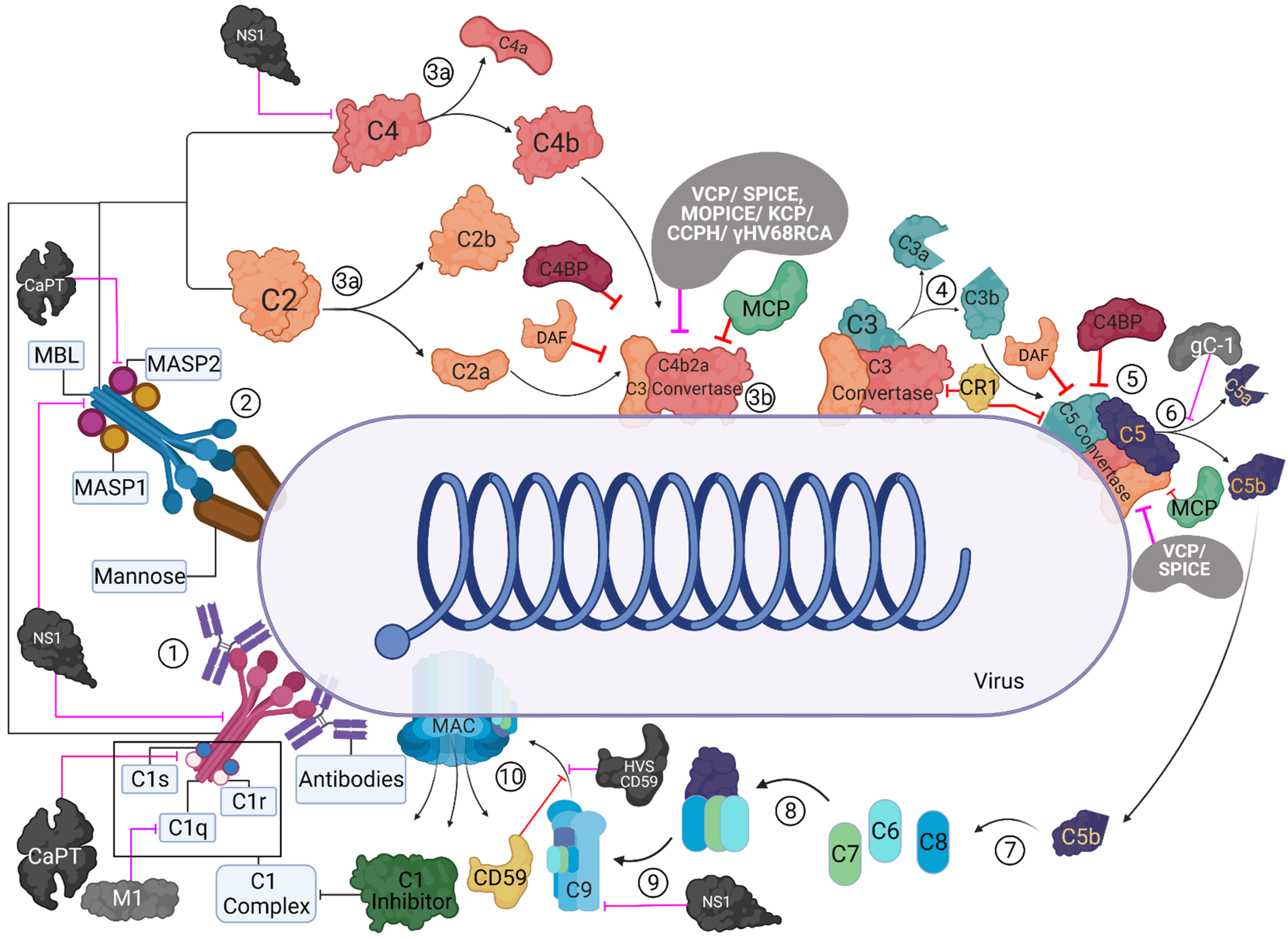
Viruses | Free Full-Text | Complement Proteins as Soluble Pattern Recognition Receptors for Pathogenic Viruses | HTML

A Novel Therapeutic Strategy for Cancer Using Phosphatidylserine Targeting Stearylamine-Bearing Cationic Liposomes: Molecular Therapy - Nucleic Acids
How well can density functional theory and pair-density functional theory predict the correct atomic charges for dissociation an

Direct versus Hydrogen‐Assisted CO Dissociation on the Fe (100) Surface: a DFT Study - Elahifard - 2012 - ChemPhysChem - Wiley Online Library

PDF) Density functionals that are one- and two- are not always many-electron self-interaction-free, as shown for H-2(+), He-2(+), LiH+, and Ne-2(+)

Faster Dissociation: Measured Rates and Computed Effects on Barriers in Aryl Halide Radical Anions | Journal of the American Chemical Society

Density functionals that are one- and two- are not always many-electron self-interaction-free, as shown for H2+, He2+, LiH+, and Ne2+: The Journal of Chemical Physics: Vol 126, No 10

Faster Dissociation: Measured Rates and Computed Effects on Barriers in Aryl Halide Radical Anions | Journal of the American Chemical Society
![PDF] Hydrogen Molecule Dissociation Curve with Functionals Based on the Strictly Correlated Regime. | Semantic Scholar PDF] Hydrogen Molecule Dissociation Curve with Functionals Based on the Strictly Correlated Regime. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/44d706e1233349d96782915ff4b7258f2aafc782/24-Figure5-1.png)
PDF] Hydrogen Molecule Dissociation Curve with Functionals Based on the Strictly Correlated Regime. | Semantic Scholar

Cation−π Interaction: Its Role and Relevance in Chemistry, Biology, and Material Science | Chemical Reviews
Chemical potential, derivative discontinuity, fractional electrons, jump of the Kohn–Sham potential, atoms as thermod

Formation of the Charge‐Localized Dimer Radical Cation of 2‐Ethyl‐9,10‐dimethoxyanthracene in Solution Phase - Choi - 2019 - Chemistry – A European Journal - Wiley Online Library



